Laboratory disposables
Sigma Fecal Transwab
Fecal Transwab® introduces a new method of collecting and transporting stool specimens that is both compatible with automated processing systems while offering faster turn round for manual processing.
Downloads
BrochureSigma Fecal Transwab
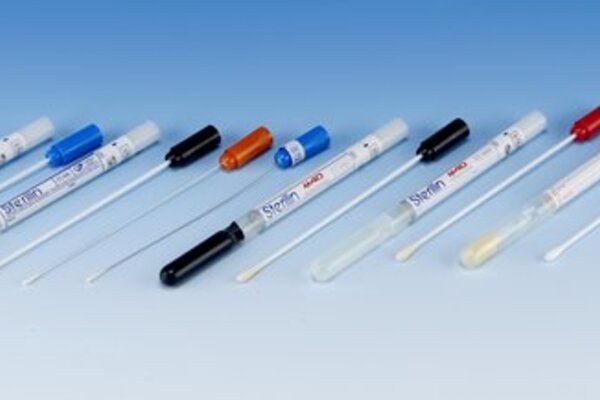
Liquid culture swabs from MWE called ∑-Transwab® are liquid culture swabs for automated and conventional processing of swab specimens.
This new specimen collection device which is M40-A Compliant, incorporates a screw cap tube and Liquid Transport Medium. The specimen is collected using ∑-Swab®, an open celled, polyurethane foam-tipped swab which allows complete flow through of reagents and micro-organisms. The specimen is then placed into the tube of liquid medium for transportation to the laboratory.
The micro-organisms in the specimen are dispersed through the medium, producing a uniform suspension ready for use, either conventionally or on an automatic specimen processing platform, or direct with many rapid molecular tests currently available.
Description
Fecal transport swab: Fecal Transwab® introduces a new method of collecting and transporting stool specimens that is both compatible with automated processing systems while offering faster turn round for manual processing.
Recommended applications
Fecal specimens.
Features
- The collection kit features a rectal swab (new design) together with a vial of liquid Cary Blair transport medium, in an easy open peel pouch.
- The collection vial contains 2ml of Cary Blair medium, specifically developed for the collection and transport of enteric microorganisms.
- The leakproof vial features a secure screw cap with integral swab capture, compatible with automated de-capping systems.
Benefits
- Liquid medium formatis compatible with automated processors, andmore convenient for manual processing.
- Integral swab capture in cap.
- High absorbency cellular flow through foam bud.
Easy to use
- Directly as a rectal swab. The highly visible red marker line indicates the safe limit for swab insertion.
- Alternatively the swab can be used to collect material from a stool sample.
When the cap is screwed on, the swab is “captured”, and remains securely fixed when the cap is removed in the laboratory, whether manually or by automatic de-capper.
Study Shows Fecal Transwab® Improves Sensitivity of Multiplex for Salmonella
A study being presented at ECCMID 2019 demonstrates that using Fecal Transwab® as the collection device improved the sensitivity of a leading multiplex based test for enteric pathogens. This study compared the results obtained when testing 38 known positives of various gastrointestinal pathogens on the Serosep EntericBio® work station using the officially cleared device (Copan FLOQswab™) and Fecal Transwab®. Concordant results were obtained for five organisms, but Fecal Transwab® demonstrated superior sensitivity for Salmonella.
Fecal Transwab® is CLSI M40-A2 compliant for culture, and has also been demonstrated to give excellent performance with many molecular platforms for bacteria, viruses and faecal parasites. The collection device includes liquid Cary Blair medium which promotes recovery of enteric pathogens without overgrowth of the inevitable commensal organisms in faecal specimens.
Note: FLOQswab™ is a trademark of Copan Italia SPA. EntericBio™ is a trademark of Serosep Ltd.
| Colour | Blue cap |
| Length | 2ml |
| Quantity | 1 |
| Pack size | 125 |
| Shelf life | 2 years |
| Normes | M40-A Compliant |
Products in this assortment
- Petri dishes (4)
- Swabs (8)
- Storage (1)
- Tubes (2)
- Containers (4)
- Cell culture (1)
- Wash bottle (1)
- Cryogenic vials & 2D microtubes (21)